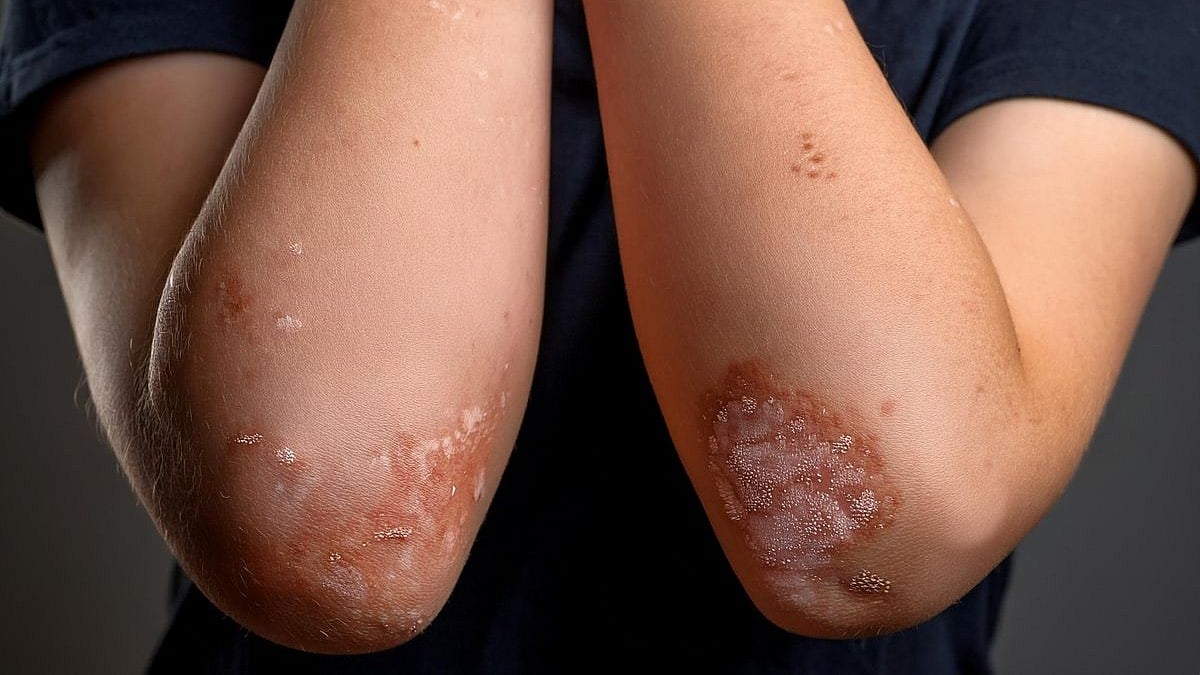
A chronic inflammatory disease that affects the pilosebaceous unit, hidradenitis suppurativa (HS) is typified by recurrent inflammatory nodules, abscesses, draining skin tunnels, and scar formation. Usually, HS affects intertriginous areas like the groin, axillae, perianal region, perineum, and inframammary regions. 1 The prevalence of HS ranges from less than 1% to 4%, 2 , 3 but it often results in pain, odor, discharge, and disfigurement, severely impacting patients’ psychological health and quality of life. 4 Not all of the pathophysiology of HS is known; however, genetic predisposition, hormonal factors, follicular occlusion, follicular rupture, and associated immune responses appear to play crucial roles in its development and progression. 5 , 6 , 7 , 8
In recent years, several epidemiological studies have identified a strong correlation between HS and cardiovascular diseases (CVDs). A meta‐analysis evaluating the relationship between HS and cardiovascular risk factors found that, compared to a control group, individuals with HS had a markedly higher incidence of cardiovascular risk factors, including obesity, smoking history, diabetes, and metabolic syndrome. 9 A study involving 5,964 patients with HS and 29,404 controls demonstrated that, after controlling for confounding factors such as obesity, smoking, and alcohol dependence, the risk of myocardial infarction (MI), ischemic stroke (IS), and cardiovascular‐related death was markedly increased in patients with HS. 10 Research from the Hidradenitis Suppurativa Foundation in the United States and Canada also recommends screening patients with HS for CVDs, with a recommendation grade of IIB. 11 Nonetheless, studies exploring the potential causal relationship (CR) and pathological mechanisms between HS and CVDs remain limited, necessitating further investigation.
Observational studies traditionally test scientific hypotheses by comparing differences in outcome distributions between high and low exposure levels rather than through interventional exposure. However, reverse causality and other possible confounding variables could impact our findings. Using genetic variants as instrumental variables to determine the strength of the CR between exposure and result, Mendelian randomization (MR) is a genetic variable analysis technique that complies with Mendel’s genetic law. 12 MR employs genetic tools for instrumental variable (IV) analysis, and an individual’s genotype is determined at conception and remains unchanged. Consequently, in contrast to retrospective research, MR lessens the interference of confounding factors and avoids reverse causality. 13 In recent years, with the increasing sample size of genome‐wide association studies (GWAS) data, MR analysis has gained popularity as an effective method for inferring causality and has been widely applied. This analytical method has revealed CRs between HS and inflammatory bowel disease, gut microbiota, and atopic dermatitis. 14 , 15 , 16 This study employs large‐scale GWAS summary data combined with MR analysis to identify the CR between HS and CVDs.
2. METHODOLOGIES AND MATERIALS
2.1. Study design
To explore the possible CR linking HS and CVDs, we employed MR analysis. In this approach, HS was considered the exposure variable, and single nucleotide polymorphisms (SNPs) strongly linked to HS were utilized as instrumental variables (IVs). The outcomes of interest encompassed five distinct CVD: coronary artery disease (CAD), myocardial infarction (MI), coronary atherosclerosis (CA), ischemic stroke (IS), and chronic heart failure (CHF). Subsequently, the roles of exposure and outcome data were reversed for a bidirectional analysis. This study adhered to three key assumptions: ① IVs should be highly correlated with exposure; ② IVs should not be influenced by variables that may potentially confuse the relationship between exposure and outcome; and ③ IVs should only affect the outcome as a direct result of exposure, without other pathways. The analytical procedure is depicted in Figure 1.

Leave a Reply