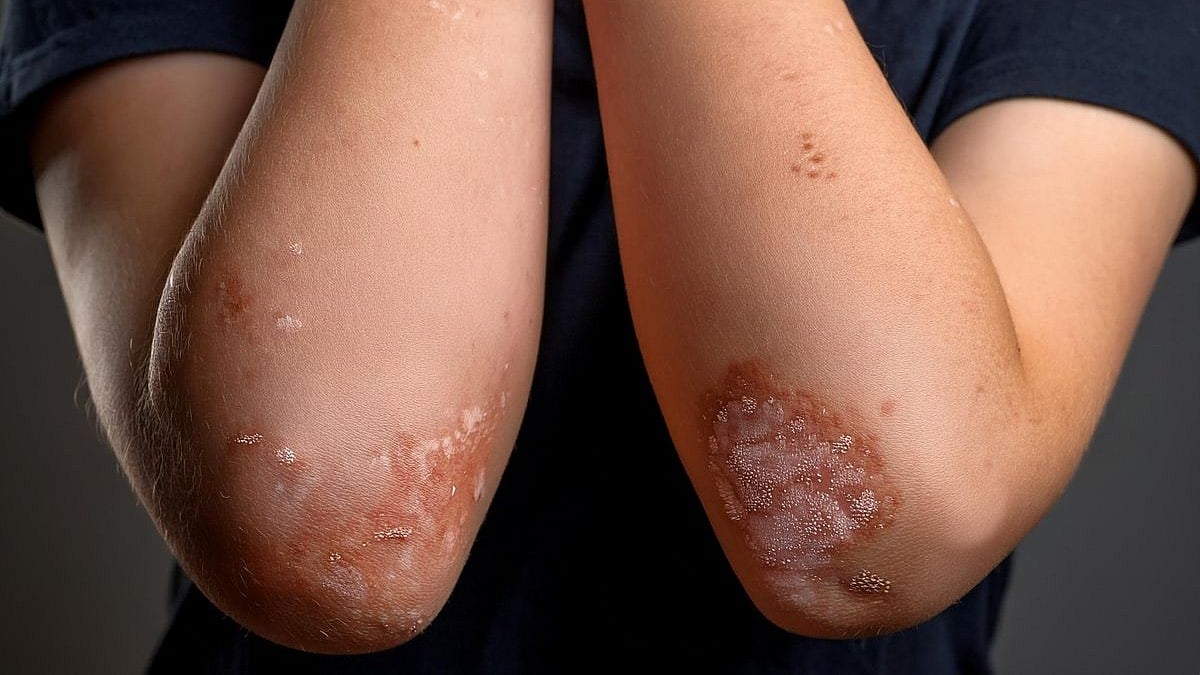
Hidradenitis suppurativa (HS), or acne inversa is a chronic, painful inflammatory disease characterized by deep-seated, painful nodules, abscesses, and draining tunnels in the apocrine gland-bearing regions of the body.1,2 Until recently, HS was considered a rare disease; however, we now know that prevalence is at least 1% in the United States and is seen at variable rates across geographical regions, and the onset of the disease typically occurs after puberty.3–6 HS disproportionately affects African Americans in prevalence and severity and affects women to men at a 3:1 ratio.4,7–10 Individuals with HS often have a poor quality of life, and are often of a low socioeconomic status, with many patients unable to work due to the severity of their disease or having lower productivity than their peers.11–15
HS is diagnosed clinically and varies greatly in presentation and many scoring systems exist to characterize disease severity.13 Because disease presentation varies, there is often a significant delay from the onset of symptoms to disease diagnosis, approximately 7 to 10 years on average.11,14 HS therapeutic options are often based on disease severity and include topicals, biologics, and surgical options.6,11 HS is a disease with many data gaps in regard to knowledge of disease pathogenesis, effective treatment, as well as accurate and timely diagnosis.15
HS patients are suspected of having a genetic predisposition along with environmental or lifestyle factors contributing to the development of disease, with smoking and obesity being the most common.1,2,4,16–18 Approximately 30% of patients reported having a family history of HS and in a large-cohort twins study, the narrow-sense heritability of HS was 77%.19 Several studies have shown that gamma-secretase mutations may be associated with the pathogenesis of HS.20,21 The purpose of this targeted review is to focus on the genetic factors described in the literature associated with HS.
Methods
Articles published until March 9, 2023, were identified in PubMed using the following search terms: hidradenitis suppurativa and gene* or acne inversa and gene*.
Articles were included if they mentioned specific genetic mutations found to be present in individuals or families with HS or acne inversa and were published in English. This search yielded 786 articles and 296 duplicates were removed, leaving 490 articles that were assessed for eligibility. Articles that did not identify specific genetic mutations were excluded from the review, of which 44 studies met the inclusion criteria (Fig. 1).22 Literature reviews were excluded from this review; however, they were referenced for background information.
Pathophysiology of HS
HS is a complex disorder involving both environmental factors, such as smoking, obesity, and hormones, as well as genetic factors; however, the exact pathophysiology is not well understood.23 A dysregulated innate and adaptive immune system has been observed in individuals with HS, with upregulation of proinflammatory cytokines and chemokines.24–26 There is still much to understand regarding the genetic factors associated with HS. Gamma secretase is important in the regulation of normal immune function and the maturation of hair follicle cells.11 Gamma-secretase complex (GSC) is a multisubunit, membrane-embedded, protease complex consisting of the following subunits: presenilin-1 (PSEN1) or presenilin-2 (PSEN2), presenilin enhancer gamma-secretase subunit (PSENEN), Nicastrin (NCSTN), anterior pharynx defective 1a or anterior pharynx defective 1b.21 GSC is involved in one of the cleavage steps that release Notch, which is required for activation of the Notch pathway. GSC and Notch are known to play an important role in the formation of epidermal cysts and comedones and dysregulation has been shown to increase the formation of these structures in HS patients.21 There have been many case studies reporting mutations in the GSC both in families with HS as well as sporadic cases.21 Additional case studies report HS in conjunction with other autoinflammatory disorders.27 Based on the review of the literature, there seem to be 3 distinct genetic classifications of HS: familial, sporadic, and syndromic.
Genetic studies
In 1968, Knaysi et al.28 conducted a review of 45 subjects who underwent surgical intervention for the treatment of HS between 1932 and 1965 at Presbyterian Hospital, Columbia University, New York, and recorded a family history of HS in 3 of 18 subjects who were specifically asked, however, no additional information was provided. Fitzsimmons et al.29 evaluated the transmission history of HS in 21 English patients across 3 families in 1984 and was the first study to suggest transmission through a single gene with autosomal dominant inheritance. A year later the same group of researchers expanded the study to 62 HS patients across 23 families, where 11 families demonstrated transmission through several generations, consistent with single gene or Mendelian inheritance.30 As seen in the previous study, 3 of the families had a familial occurrence, while 9 of the families showed no family history at the time of the study, which the team noted may be due to inaccurate diagnosis and false negative family history.30 Fifteen years later, Von der Werth et al.31 set out to test the validity of the Fitzsimmons et al. study and reviewed the 14 surviving probands and their families, where they saw the same autosomal dominant inheritance pattern seen in the previous study in those where a positive family history had been noted previously.
Familial HS
Approximately 30 to 40% of individuals with HS report having at least one affected first-degree relative, indicating genetics are likely involved in the pathogenesis.11 In 2006, Gao et al.10 mapped the candidate HS gene to chromosome 1p21.1–1q25.3 by genome-wide linkage analysis in a 4-generation Chinese family, though no specific gene was identified due to the large genomic region. Another study conducted a few years later evaluated 29 individuals from 2 large, multigenerational families with HS by linkage analysis with the 9 satellite markers used in the previous study.5 The results of this study ruled out 1p21.1–1p25.3 involvement in the pathogenesis of HS in the 2 families evaluated; however, HS presented as an autosomal dominant trait with 100% penetrance with no skipping of generations observed.5
Wang et al.32 was the first group to report mutations in the GSC in 6 Han Chinese families; families 1 and 2 had different PSENEN mutations, while families 3–6 had either NCSTN or PSEN1 mutations. In families 3–6, reverse transcription polymerase chain reaction demonstrated a reduction in transcript expression in individuals with a frameshift or nonsense mutation in PSEN1 or NCSTN, indicating these mutations ultimately lead to a loss of function, leading to defective Notch signaling.32 Twenty-five familial HS case studies were reviewed and revealed 37 unique mutations in the GSC (Table 1). Mutations in NCSTN were present in all affected family members of 31 families, while mutations in PSENEN were present in all the affected family members of 5 families and mutations in PSEN1 in one family; mutations in these genes were not seen in the family members and control subjects without HS. The cases reviewed exhibited autosomal dominant inheritance with incomplete penetrance. This data indicates that mutations affecting GSC are involved in some familial HS cases; however, there are likely other genes involved in the molecular pathogenesis of HS that have not yet been identified.


Leave a Reply