Hidradenitis suppurativa (HS), also known as acne inversa or Verneuil disease, is a chronic and debilitating inflammatory skin condition typically involving the hair follicle of apocrine gland-bearing skin [1,2]. It usually appears after puberty, especially during the third decade [3], with a typical recurrent and progressive course, which leads to a profound impact on the patient’s quality of life [4].
Depending on the severity and the stage of the disease, patients with HS may present with a variety of skin lesions, ranging from papules and pustules, comedones and inflamed cysts to recurrent painful, deep-seated nodules and abscesses, which may develop into draining sinus tracts and hypertrophic scars [5].
Lesions typically appear in the intertriginous areas, with a preferential distribution on the axillary, inguinal and submammary folds in females, whereas the perianal area and buttocks are more commonly involved in males [6,7,8]. In addition, patients may experience malodorous discharge, disfigurement, itching and pain [9], with a significant emotional impact and social isolation due to fear of stigmatization [10,11]. The global prevalence of HS ranges from 0.00033 to 4.1% (most likely 0.7–1.2% in the European and US populations) [5]. Despite the high burden of the disease on patients, the therapeutic options for HS are currently limited. Adalimumab (anti-TNF-α monoclonal antibody(mAb)) [12], secukinumab ((mAb targeting interleukin(IL)-17A) [13] and bimekizumab (mAb selectively inhibiting both IL-17A and IL-17F) [14] are the only approved biologic agents for the treatment of moderate-to-severe HS and provide a clinical benefit in approximately 40% of HS patients, still resulting in a high unmet clinical need.
The pathogenesis of HS is largely unknown, resulting from a complex interplay of genetic predisposition, smoking, environmental factors and innate and adaptive immune dysfunction [15]. Increasingly, studies have supported the primary role of innate immunity dysregulation in HS, and thus of autoinflammation. Autoinflammation involves a predominant dysregulation of innate immune responses, in some cases caused by an overactivation of the inflammasome, a multiprotein cytosolic complex responsible for sensing cellular stressors [16]. Also, the primary role of innate immunity dysregulation and autoinflammation in HS is supported by its presence in the context of autoinflammatory diseases (AIDs), such as PASH (pyoderma gangrenosum (PG), acne and HS) and PAPASH (pyogenic arthritis, acne, PG and HS) syndromes [17]. Regarding this background, understanding the pathogenetic mechanisms of HS could shed light on new and unexplored molecular pathways also shared by AIDs conditions and pave the way for new targeted therapeutic strategies.
Finally, according to the current literature and ongoing research, HS should be regarded as a potential subtype of autoinflammatory keratinization disease (AiKD), a recent disease entity proposed by Akiyama et al. [18]. AiKDs are characterized by the following three criteria: (i) the primary sites of inflammation are the epidermis and the upper dermis; (ii) inflammation leads to hyperkeratosis, which is the main and characteristic phenotype of AiKDs; and (iii) AiKDs have primary genetic causative factors associated with the hyperactivation of innate immunity (autoinflammation), mainly in the epidermis and upper dermis [19].
Thus, the role of this review is to thoroughly explore the current scientific evidence underlying the role of autoinflammation in HS, ranging from genetic data to molecular studies and clinical evidence, as well as to provide a forward-looking update on the relationship between HS and AIDs.
2. Pathogenesis of Hidradenitis Suppurativa
For years, HS has been considered a bacterial skin infection of the terminal follicle, resulting in an intense and destructive local inflammatory response due to its typical clinical features, such as purulent discharge in apocrine gland-bearing areas and clinical benefit with antimicrobial treatment [20]. However, microbiologic screening of purulent discharge usually reveals negative cultures or a mixture of normal flora and skin commensals, with no specific growth of bacterial agents in culture to date [21]. The role of bacteria remains controversial as it has been found that the microbiome in HS lesional and non-lesional skin differs from healthy controls, supporting the hypothesis of a link between a dysbiotic cutaneous microbiome and HS lesions [22].
Although biopsies are not routinely performed for diagnostic purposes, the histopathologic examination of lesions is certainly helpful in understanding the events underlying the pathogenesis of HS: typical histological patterns are hyperkeratosis of terminal follicular openings, hyperplasia of the follicular epithelium and perifolliculitis with immune cell infiltration [23].
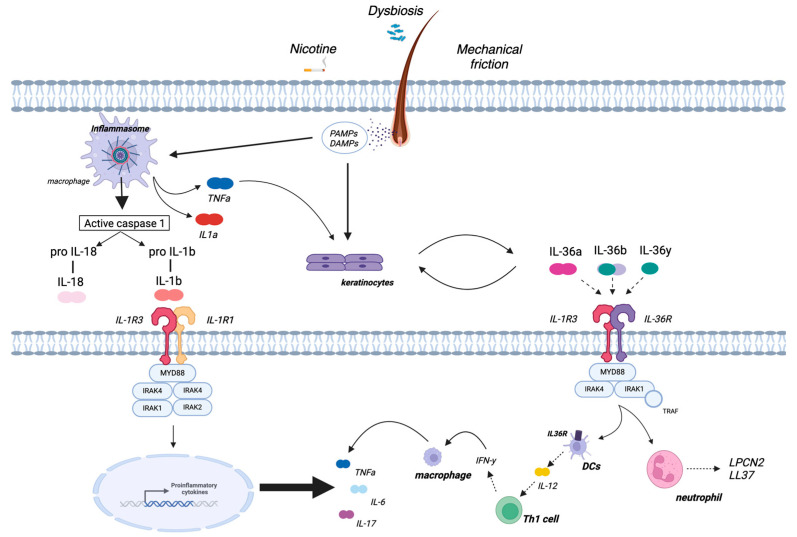
A key pathogenic event in HS is now considered to be the hyperkeratotic plugging of the terminal hair follicle opening, with consequent occlusion and dilatation/elongation of the hair follicle, leading to rupture of the follicular epithelial wall and bacterial biofilm formation [21]. These events trigger the immune response and result in the release of Damage and Pathogen Associated Molecular Patterns (DAMPs and PAMPs, respectively) and consequent activation of innate immunity. In detail, resident immune cells, such as macrophages, are hyper-responsive to PAMP stimulation in HS due to an increased expression of toll-like receptor 2 (TLR2) and secrete pro-inflammatory cytokines, including TNFα, IL-6 and IL-1β [24]. The inactive precursor of IL-1β (pro-IL-1β) is converted into the active form IL-1β by a cleavage process mediated by a multiprotein complex called the inflammasome [25]. Therefore, over-activation of the innate immune system appears to be central, especially in the early phase of the disease, due to the massive production of inflammatory cytokines in response to dermal seeding of keratin, sebum, bacterial components and cellular debris [26]. Indeed, according to several studies, one of the most prominent immunological features observed in HS-injured skin is the upregulation of TNF-α and IL-1β pathways, mainly secreted by macrophages [23,27,28,29]. These cytokines induce the expression of a broad range of chemokines, including CXCL8, CXCL11, CCL2 and CCL20 in keratinocytes and CXCL1 and CXCL6 in fibroblasts, bolstering further infiltration of immune cells [27]. This process includes granulocytes, T cells, B cells and monocytes, which locally differentiate into macrophages and dendritic cells, and enhances the fibroblastic secretion of matrix metalloproteinases (MMP), which are involved in tissue destruction and tunnel formation [27]. In addition, activated keratinocytes release pro-inflammatory cytokines such as IL-1β, IL-36α, IL-36β and IL-36γ (all of the IL-1 cytokine family) [30]. These cytokines target different cell types, from keratinocytes to dendritic cells, and promote the production of pro-inflammatory cytokines such as IL-12 and IL-23, which induce adaptive immunity (Th1 and Th17 responses) [31]. Dendritic cells also produce IL-36, creating an autocrine loop that further amplifies inflammation [30]. In the chronic phase of HS, an imbalance between Th17 cells and regulatory T cells (Tregs) is found, with elevated serum levels of IL-17 and a local increase in the ratio of proinflammatory Th17 cells and Tregs [32]. The enrichment of Th17 cells in HS skin and the dysregulation of the Th17:Treg cell axis are likely driven largely by increased IL-1β and IL-6 because of innate immunity overactivation [33]. A schematic representation of HS pathogenesis is provided in Figure 1.
3. Preclinical Evidence on the Role of Autoinflammation in Hidradenitis Suppurativa
To date, there is a wealth of preclinical scientific evidence, ranging from genetic to molecular, underscoring the role of autoinflammation in HS [34].
The importance of genetic factors in HS is well known and established, with approximately 35–40% of patients having a family history of HS [35,36].
In 2010, heterozygous loss-of-function mutations in NCSTN, PSENEN and PSEN1 were identified in six Chinese patients with HS [37]. These genes are known to encode for components of γ-secretase, a multi-subunit complex involved in the signal transduction associated with NOTCH receptors, which regulate both proliferation and differentiation of keratinocytes [38].
Interestingly, not only are genes involved in keratinization processes implicated in HS and its syndromic forms but also those related to autoinflammation [39]. Specifically, genes associated with HS can be categorized into two distinct groups. The first group includes NCSTN, PSENEN, PSEN1, POFUT1, POGLUT1, KRT17, KRT6A, FGFR2 and GJB2. Mutations in these genes result in autoinflammation, which is preceded by keratinization. It has been proposed that this HS subtype could be regarded as an AiKD in the broad sense. The second group of genes includes MEFV, NOD2, LPIN2, NLRP3, NLRP12, PSMB8, MVK, IL1RN and PSTPIP1, whose mutations lead to keratinization preceded by autoinflammation. Conversely, this HS subtype could be regarded as an AiKD in sensus stricto, as it is most likely the autoinflammation that is at the heart of the pathogenic process [39].
In detail, the PSTPIP1 gene encodes the enzyme proline–serine–threonine phosphatase-interacting protein 1 (PSTPIP1) [40]; mutations that cause its overactivity lead to its hyperphosphorylation and increased assembly of the pyrin inflammasome, ultimately resulting in the uncontrolled release of IL-1β [40]. Additionally, the MEFV gene encodes for pyrin, a clue component of the pyrin inflammasome [41]. HS may affect patients with familial Mediterranean fever (FMF), a prototypic monogenic AID, carrying MEFV mutations, with a higher frequency of MEFV mutations reported in HS patients than in healthy controls, suggesting a potential contribution to HS pathogenesis [42,43].
Furthermore, mutations in MEFV, NLRP3, NLRP12, NOD2 and LPIN2 have been investigated in genomic DNA samples from patients affected by pyoderma gangrenosum (PG) and its syndromic variants, including also HS in their spectrum, which will be discussed in greater detail later in this review [44].
Based on genetic evidence confirming the autoinflammatory nature of HS, several experimental studies have thoroughly investigated the role of the IL-1 family, a key element of innate immunity, in HS [45,46].
Specifically, gene expression analysis of immune cells isolated from HS skin revealed an overexpression of B cells, antimicrobial peptides and IL-17A, along with enrichment of IL-1-related pathways and IL-17A-producing Th17 cells in HS skin compared to healthy skin [47]. In another study, expression levels of IL-1 pathway mediators were quantified in lesional HS skin samples in comparison to healthy donor skin samples, using real-time PCR (RT-qPCR) [27]. IL-1β was the most expressed cytokine in HS lesions compared to healthy skin, and its expression was even more pronounced than in other inflammatory skin diseases such as psoriasis. Moreover, IL-1β levels were found to be elevated in perilesional HS skin [27].
As IL-1β is processed by caspase-1, Kelly et al. [28] employed flow cytometry using the caspase-1 fluorochrome inhibitor of caspases (FLICA) to detect active caspase-1 in skin biopsies. The results showed that caspase-1 activation was enhanced in live cells from HS lesional skin compared to cells from perilesional skin. Furthermore, caspase-1 inhibition was found to partly suppress IL-1β secretion in HS lesional skin, indicating a high expression of caspase-1 in HS lesions [28].
Not only IL-1 but also IL-36 cytokines have been studied in HS. The IL-36 subfamily members belong to the IL-1 family and have been demonstrated to play a role in HS pathogenesis [48]. In 2017, skin biopsies were collected from the affected sites of HS patients for in vivo mRNA investigation of IL-36α, IL-36β, IL-36γ and IL36Ra by RT-PCR. Increased mRNA expression of IL-36α, IL-36β and IL-36γ was found in the lesional skin of HS patients as well as in patients affected by acne, suggesting a common pathogenesis of the two skin conditions. Conversely, no increase in IL-36Ra, which is an anti-inflammatory member of the IL-36 subfamily, was detected. Furthermore, the expression of IL-8 was investigated in skin conditions such as acne, HS and psoriasis. A significant increase was observed in all settings, with the highest level of IL-8 expression observed in HS and the lowest in psoriasis [48]. These results were corroborated by another study, performing immunostaining on skin biopsies of inflammatory HS lesions. The expression levels of IL-36α, 36β and 36γ were all significantly higher in lesional and perilesional HS skin than in healthy controls. Furthermore, immunohistochemical analysis revealed a primary expression of IL-36 cytokines by keratinocytes, highlighting a pivotal role of the IL-36 family in the crosstalk between keratinocytes and immune cell activation and, in turn, in determining the outbreak of HS lesions [49]. Finally, systemic levels of IL-36 were investigated in healthy donors, HS and psoriatic patients’ serum through ELISA. Patients affected by HS exhibited elevated levels of the isoforms IL-36α, IL-36β and IL-36γ, with no detection of IL-36RA [50].
Recently, Moran et al. [51] employed single-cell RNA sequencing (scRNA-Seq) to investigate CD45+ cells isolated from healthy control skin and HS surgical resection tissue. Myeloid clusters in HS skin showed significantly higher levels of NLRP3, IL-1β and IL-18 in HS samples compared to the controls. It is noteworthy that a correlation between IL-1β and Th17-mediated inflammation was revealed, thereby confirming the role of IL-1 in downstream inflammation in the pathogenesis of HS [51].
From a mechanistic perspective, certain monogenic AIDs result in the sustained activation of the NLRP3 or pyrin inflammasomes, which subsequently activate caspase-1, leading to the release of IL-1 and the onset of autoinflammation. Indeed, alongside an increase in IL-1β, elevated levels of caspase-1, NLRP3, IL-6 and IL-18 have been reported in the lesional skin of HS patients [51,52].
Interestingly, an ex vivo explant model was recently used to culture skin in the presence or absence of the prototypical NLRP3 inflammasome inhibitor MCC950. Administration of MCC950 determined a consistent decrease in the levels of IL-1β, TNF-α, IL-17A, IFN-γ, CCL20, CXCL1, IL-8 and IL-36γ from involved HS skin, with no significant change in the release of IL-1α, IL-18, IL-17C, MMP3 or S100A8. This confirms the pivotal role of the NLRP3 inflammasome in shaping the inflammatory burden in HS-affected skin as well as the potential of its blocking in HS treatment [52].




Leave a Reply